All right, back with another fundamentals post.
The basics are what provide the highest ROI in anything.
So let’s get into the meat of it.
Elements: Carbon
For today:
Let’s use the element Carbon for The Periodic Table!
All of these numbers and letters are not that complicated.
It’s just a matter of defining what they mean.
At the top is the name “Carbon”.
But at the bottom of the picture, it says “Carboneum”.
You can ignore “Carboneum”, it is just the Latin name for “Carbon.”
The big letter “C” is the atomic number symbol of the element.
Carbon → C.
The “6” next to the “C” is the atomic number.
The atomic number is the number of protons in the atom. The atomic number is only for protons (positively charged atoms).
So there are 6 protons in the carbon atom.
The number “12.011” is the atomic mass relative to the atom.
Atomic Mass Relative is the mass of an atom of an element,
That is in comparison to its most commonly found isotope.
In this case, Carbon’s most abundant isotope is Carbon-12.
Carbon is normally found as Carbon-12,
An isotope of carbon that consists of six protons & six neutrons.
This is where Carbon-12 gets the number “12” from.
But because of this,
You could say that Carbon has 12 units, or 1/12 the mass of Carbon-12.
The number “2.55” is the electronegativity of the Carbon element.
Electronegativity is the chance that a pair of electrons will be attached to a certain atom.
If the difference between the electronegativity is greater or equal to 0.5:
The molecule is polar.
If the difference in electronegativity in the molecule is less than 0.5:
The molecule is non-polar.
Covalent bonds occur at an electronegativity below around 1.7.
Ionic bonds occur at an electronegativity equal to or greater than 1.7.
(If you want to know more about electronegativity, I will link my articles below to follow up on the concepts.)
Next-Level Concepts
Now that we’re clear on the fundamental concepts of the element “Carbon”,
Let’s proceed with the more complicated numbers and symbols.
The number “3550°” is the melting point of Carbon in Celcius.
In Fahrenheit, the melting point is 6332°F,
In Kelvin, the melting point is 3773.15 K.
The number “4827°” is the boiling point of Carbon in Celcius.
In Fahrenheit, the boiling point is 8720.6°F,
In Kelvin, the boiling point is 5100.15 K.
The number “2.267” is the density of the element in grams/centimeter cubed (g/cm³).
On the left of the element, there are the numbers (-2, +2, +4) in a graph.
These numbers are the potential oxidization states of the element carbon.
If it has a negative number:
The carbon element has gained electrons from another element.
If it has a positive number,
The carbon element has lost electrons from another element.
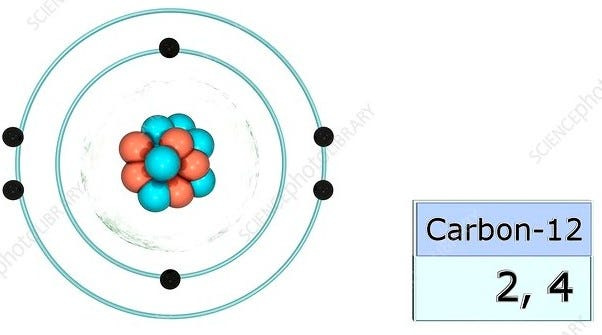
On the right of the element, there are the numbers (4, 2) in a graph.
These numbers are the distribution of electrons in the carbon atom.
The order of the numbers starts from the outer electron shell to the inner electron shell.
Here is a photo of the distribution of electrons in a carbon atom if you’re confused:
Finally, there are the numbers and letters (1s², 2s², 2p²) below the atomic number symbol “C”.
These are the electron configurations of the carbon atom.
An “S” Orbital looks like a circle.
It is a circle because all of the electrons in that orbital are filled.
An “S” orbital is known as a Sigma Bond.
A “P” Orbital is a little bit different.
There are three ways “P” orbitals can be found:
“x-axis”, “y-axis”, and “z-axis” orbitals.
A “P” bond is known as a Pi Bond.
Finally, an “S” electron configuration bond can hold up to two electrons.
A “P” electron configuration bond can hold up to six electrons.
Done.
We tend to fear what we don’t know.
But once we know, we feel like fools.
For giving in to the cowardice, the precautions.
See you next time for reading oxidation.
With energy,
Carlos ⚛️
Know a materials science fanatic?